Stay Informed
Follow us on social media accounts to stay up to date with REHVA actualities
|
|
|
Elena Piecková | Renáta Lehotská | Soňa Wimmerová |
Faculty of Medicine, Slovak Medical University, Bratislava, Slovakia | Faculty of Medicine, Slovak Medical University, Bratislava, Slovakiarenata.lehotska@szu.sk | Faculty of Public Health, Slovak Medical University, Bratislava, Slovakiasona.wimmerova@szu.sk |
Visually clean air contains denser bioaerosol than the dirty one as bigger propagules sediment faster. Airborne fungi are commonly forming conglomerates similar to fine fog (10 – 20 µm in diameter) [1].
Employees dealing with material rich in nutrients and prone to fungal colonization due to damp conditions might be exposed to extreme fungal concentrations (109 colony forming units, cfu/m³) – condition known as “particle burst”, and plenty of mycotoxins. Inhalation of pathogen / toxicant / irritant may result in health damage at 100-times lower loads than after ingestion, due to the crossing over even haemo-encephalitic barrier [2]. Inhalation exposition to mycoaerosol is not a part of routine analysis of indoors yet [3, 4].
The study on quantitative aeromyco-analysis of places with relevant historical artefacts with estimation of possible fungal load to the individuals is presented.
Nine localities of running research works with human remains or of their public expositions in Slovakia and Hungary are observed from 2012 onward. Indoor and related outdoor air in mausoleums, depositaries, crypts, reliquaries, museums and an archive, as given in the Figure 1, was sampled by mean of an impactor. The DG18 agar (HiMedia, Mumbay, India) was employed as the isolation medium for 67 complex air samples, incubated at 25 and 37 °C 3 – 7 days as recommended by the IUMS Committee on Environmental Mycology. The average cultivable fungal load in cfu/m³ was calculated and statistically processed by the pair t-test.
Experts working at the sampling places and visitors are groups of interest from the mycoaerosol exposition point of view. Esp. the later ones might be in health conditions when being more sensitive to health damage due to fungal bioaerosol, incl., fungal toxic products, e. g. allergic, elderly or polymorbid persons. Quantities of cultivable aeromycobiota are summarized in the Figure 1.
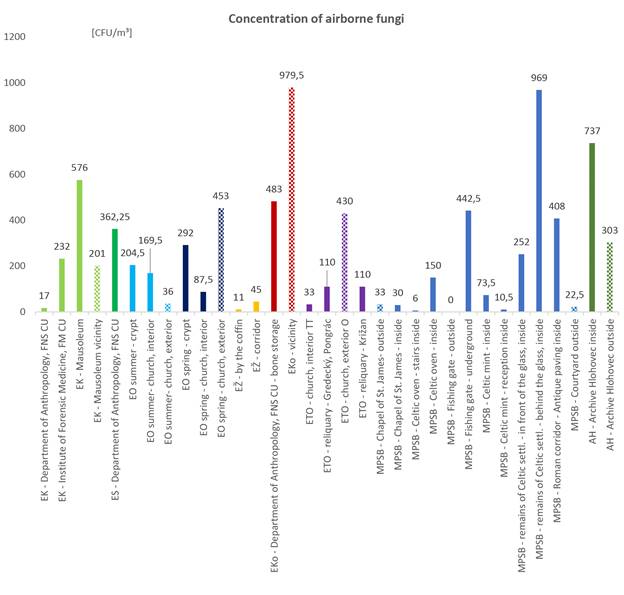
Figure 1. Quantification of cultivable airborne fungi in localities with different sampling sites. Legend: EK – Sladkovicovo, ES – Solosnica, EO – Okolicne, EŽ – Zofia Serediova, Eko – Kovarce, ETO – Trnava and Esztergom, MPSB – Bratislava, AH - Archive Hlohovec.
Presence of internal sources of fungal contamination in glass covered undergrounds without air circulation is documented in the Table 1. According to the WHO recommendation [5], the indoor fungal concentration must not exceed the outdoor one. The qualitative composition of both fungal aerosols must cope with each other. And no pathogenic and toxic fungal species are allowed indoors. If, even, one of the given conditions is missing, the indoors is classified as the sick one.
From the hygienic conditions in terms of airborne fungal content, the mausoleum yielded very high concentration, while specialized departments conducting research on the same mortal remains (the university) presented loads lower. Apparently, due to regular decontamination of the environment and handling tools. Total fungal count in the indoor air of the archive did not exceed its outdoor concentration with statistical relevance.
Table 1. Identification of indoor fungal sources in the localities with historical objects. Legend: ci – indoor air concentration of fungi (average); co – related outdoor air concentration of fungi (average); ci/co > 1 – no indoor fungal source present, ci/co > 1 – indoor fungal source likely.
Locality | ci [cfu/m³] | co | ci/co | |
Sladkovicovo | 441 | 201 | 2.2 | |
Okolicne | Summer | 187 | 36 | 5.2 |
Spring | 190 | 453 | 0.4 | |
Zofia Serediova | 11 | 45 | 0.2 | |
Kovarce | 483 | 979.5 | 0.5 | |
Bratislava | Chapel St. James | 30 | 33 | 0.9 |
Castle | 543 | 22.5 | 24.1 | |
Awad et al. [6] evaluated total indoor environment in a museum in Giza, Egypt. They found 175 - 40,250 cfu/m³ of airborne fungi. Ratio indoor/ outdoor air showed the outdoor environment was the main source of fungi isolated indoors. Concentrations of aerial mycobiota in our study fit the interval 6 – 979.5 cfu/m³. There is always a dynamic exchange between indoor and outdoor fungal bioaerosol as proved by genetic analysis [1].
The exposition was calculated as total number of inhaled propagules in one or eight hours at a normal ventilation rate of 5 – 8 litres of air per min: 5 is the value in steady state person (visitor, inhaled volume 0.3 m³) and 8 during a work shift (8 hrs, 3.84 m³) [7].
Formula:
V × C = X
V – inhaled air volume over an hour or 8 hrs [m³]
C – average concentration of airborne fungi [cfu/m³]
X – whole number of inhaled fungal propagules in the particular exposition course [cfu]
Table 2 shows the calculated fungal load in cfu inhaled over 1 hour (visitor) or 8 hours (staff member).
Table 2. Sampling locality and inhaled fungal cfu per 1 hr (visitor) or 8 hrs (worker).
Locality | C value [cfu/m³] | cfu/1 hr | cfu/8 hrs | |
Sladkovicovo | mausoleum | 576 | 173 | 2,212 |
Comenius University | 98 | 29 | 375 | |
Solosnica | 362 | 109 | 1,391 | |
Okolicne | summer | 229 | 69 | 880 |
spring | 193 | 58 | 74.5 | |
Zofia Serediova | 25 | 7 | 94.5 | |
Kovarce | 483 | 145 | 1,855 | |
Trnava | 84 | 25 | 324 | |
Ostrihom | 110 | 33 | 422 | |
Bratislava | 260 | 78 | 999 | |
Hlohovec | 303 | 91 | 1,163.5 | |
Chen et al. [9] monitored number of visitors in the Museum of Terracota Army of the Emperor Qin in China. Max indoor fungal cfu/m³ detected were 90 and was clearly related to the peaking number of tourists present in the museum.
Concentrations of fungal isolates were analysed by pair t-test with the significance level α = 0.05
Between paired localities, there were no statistically relevant differences in quantities of air fungi (p > 0.05), exc. of very complex nutrient samples from Sladkovicovo (mummies, osseous remains, rests of cloths and bandages) vs. dominating paper in the archive (p = 0.031).
Performed quantitative analysis of indoor aeromycobiota pointed out:
· high concentrations of fungi might lead to ill health of persons staying in places, where esp. mycosis outbreaks remain the less described – an infectious dose of (opportunistic) pathogenic moulds is unknown in general (even one propagule perhaps), with remarkable amounts of fungal propagules inhaled by the staff working on site for 8 hrs;
· the settled aerosolized fungi might damage historically valuable objects irreversibly and some indoor fungi (zygomycota) are early indicators of microclimatic conditions favourable to biodeterioration.
There is ongoing lack of standardized sampling methods as well as of set hygienic exposition limits to humans. The high complex bioaerosol composition and very individualized responses of human organisms being exposed might be a crucial complication. Combination of several principles in sampling is highly recommended to describe the aeromycobiome and its harming potential with the supreme objectivity.
[1] Pieckova E. Indoor microbial aerosol and its health effects: Microbial exposure in public buildings – viruses, bacteria, and fungi. In: C. Viegas, et al. (Eds.): Exposure to microbiological agents in indoor and occupational environments. Springer, Cham, (2017) 237 – 252. ISBN 978-3-319-61688-9.
[2] Pieckova E. Mycotoxins and Their Inhalatory Intake Risk. In: K. Singh, N. Srivastava (Eds.): Recent Trends in Human and Animal Mycology. Part II: Mycotoxins in Relation to Human and Animal Health. Springer (2019), pp. 195 – 202. ISBN 978-981-13-9434-8.
[3] Malta-Vacas J., Viegas S., Sabino R., et al. Fungal and microbial volatile organic compounds exposure assessment in a waste sorting plant. Journal of Toxicology and Environmental Health, Part A. 75 (2012) 1410 – 1417. ISSN 1528-7394.
[4] Viegas S., Pinheiro C., Sabino R., et al. Environmental mycology in public health. Fungi and mycotoxins risk assessment and management. Elsevier–AP, London (2015) pp. 234. ISBN-13: 978-0124114715.
[5] WHO Regional Office for Europe: WHO guidelines for indoor air quality: dampness and mould. Geneve: WHO 2009, pp. 228.
[6] Awad A., Saeed Y., Shakour A., et al. Indoor air fungal pollution of a historical museum, Egypt: a case study. Aerobiologia. 36 (2020) 197 – 209. doi: 10.1007/s10453-019-09623-w.
[7] https://en.wikipedia.org/wiki/Minute_ventilation. Online. 09/05/022.
[8] Chen Y.P., Cui Y., Dong J.G. Variation of airborne bacteria and fungi at Emperor Qin's Terra-Cotta Museum, Xi'an, China, during the "Oct. 1" gold week period of 2006. Environ Sci Pollut Res Int. 17(2) (2010) 478-485. doi: 10.1007/s11356-009-0161-1.
Follow us on social media accounts to stay up to date with REHVA actualities
0