Stay Informed
Follow us on social media accounts to stay up to date with REHVA actualities
|
|
|
|
Alireza Afshari | Olli Seppänen | Bjarne W. Olesen | Jinhan Mo |
Department of the Built Environment, Aalborg UniversityKøbenhavn SV, Denmarkaaf@build.aau.dk | Nordic Ventilation Group & FINVACHelsinki, Finland | Department of Civil EngineeringLyngby, Denmark | PhD, Associate ProfessorBeijing Key Laboratory of Indoor Air Quality Evaluation and Control, Department of Building Science, Tsinghua University, Beijing, P. R. China |
Portable gas-phase air cleaners use different technologies to remove gaseous pollutants, which include inorganic gases (e.g. carbon monoxide and nitrogen dioxide), ozone (O₃), and organic gases (e.g. volatile organic compounds (VOCs) and aldehydes). Hundreds of different gaseous pollutants have been detected in indoor air. Sources of inorganic gases include gas stoves, tobacco smoke, and vehicles. Sources of O₃ include infiltration from outdoors and O₃ generation from indoor sources, such as laser printers. Sources of organic gases include tobacco smoke, building materials, furnishings, animal metabolic processes, outdoor sources, cooking and plant products and such products as paints, adhesives, dyes, solvents, caulks, cleaners, deodorisers, cleaning chemicals, waxes, hobby and craft materials and pesticides. In addition, radon can also be found in indoor air. Portable air-cleaning devices are not effective at reducing radon levels in a building and are not recommended as a radon mitigation measure (Environmental Protection Agency (EPA), 2009).
There are six principal types of gas-phase air cleaners. The most commonly applied methods are adsorbent media air filters, such as activated carbon (AC), chemisorbent media air filters, photocatalytic oxidation (PCO), plasma, O₃ generators and plants.
Regardless of the type of technology, three requirements must be fulfilled. First, high filtration efficiency must be provided for a broad range of chemical substances. Second, low airflow resistance (small pressure drop) is required. Finally, the release or generation of harmful substances must be prevented. The performance of a portable gas-phase air cleaner depends on several factors:
· device flow rate, and velocity through the media
· filter type and efficiency,
· construction quality, which affects the air-bypass around the filter,
· gas concentration and types in the air,
· room conditions, such as air temperature and humidity, which affect the capacity of adsorbents to remove odours and chemicals, and
· unit placement in the room.
At least three primary descriptors of the efficiency of cleaning systems exist:
· the VOC degradation rate,
· the one-pass removal efficiency, and
· the clean air delivery rate [Li Puma et al., 2009; Mo et al., 2009] (initially defined by the Association of Home Appliance Manufacturers and well recognised by manufacturers), indicating the clean air volume delivered by the treatment system (usually in m³ h−¹).
This summary Table 1 is preceding the detailed descriptions and explanations regarding the different technologies to encourage the reader to dive into those details.
Table 1. Summary of the technologies.
Technology | Advantage | Disadvantage | Application |
Adsorbent | · Gaseous pollutants adsorb on porous granular media or condense in pores of media. · Many types of sorbents with activated carbon most commonly used. · Widely available technology · Can remove broad range of gaseous pollutants with moderate to high efficiency | · Pollutants can be released from sorbent into indoor air · Low effectiveness for low molecular weight pollutants including formaldehyde · Must periodically replace sorbent · Sorbent lifetime for indoor air applications not well understood · Large amount of sorbent needed for long lifetime · High sorbent cost · Often high airflow resistance increasing fan energy use | · Installed in heating, ventilating and air conditioning systems or in stand-alone portable air cleaners |
Chemisorbent | · Gaseous pollutants adsorb on and chemically react with porous granular media · Widely available technology · Can remove broad range of gaseous pollutants with moderate to high efficiency | · High chemisorbent cost · Often high airflow resistance increasing fan energy use | · Installed in heating, ventilating and air conditioning systems or in stand-alone portable air cleaners |
Photocatalytic Oxidation | · Gaseous pollutants adsorb on a surface coated with a photocatalyst that is irradiated with a light source, usually a source of ultraviolet light; some adsorbed pollutants decompose · Can remove a range of gaseous pollutants · Usually lower airflow resistance than sorbents and chemisorbents, thus, lower fan energy use · Can destroy some bioaerosols · Many systems have low pollutant removal efficiency | · Lamp energy use · Cost of periodically replacing lamps · Photocatalysts become inactive, with unknown photocatalyst life · Incomplete breakdown of some pollutants can result in formation of new pollutants potentially harmful to health | · Installed in heating, ventilating and air conditioning systems or in stand-alone portable air cleaners |
Air Ion Generators | · Radicals (small reactive molecules) created by electric discharge can oxidize and decompose volatile organic compounds and nitrogen oxides · Quiet and energy efficient · May improve particle removal performance of some particle air cleaners | · Very limited data available on pollutant removal performance in buildings · Can produce ozone, see comments on ozone air cleaners | · Usual application is a standalone portable air cleaner |
Ozone Generators | · Ozone generated and released into indoor air can react with and breakdown some airborne volatile organic compounds · Quiet and energy efficient | · Releases ozone into indoor air and ozone is a harmful pollutant · Generally ineffective in significantly reducing airborne volatile organic compounds unless ozone concentrations are very high · Reactions of ozone with airborne volatile organic compounds can lead to production of formaldehyde and ultrafine particles that pose health risks | · Usual application is a standalone portable air cleaner |
Plants | · Plants in buildings can remove some volatile organic compounds · Quiet and energy efficient | · Not proven to significantly reduce indoor pollutant levels with practical number of plants · Plants and molds on plants and soil can be a source of pollutants | · Plants placed throughout building or in attached greenhouse · One system forces air through plant root zone |
* For more information, see the ASHRAE Position Document on Filtration and Air Cleaning.
https://www.ashrae.org/file%20library/about/position%20documents/filtration-and-air-cleaning-pd.pdf
Several portable air-cleaning technologies are designed to either remove gaseous air pollutantsor convert them into harmless by-products using a combination of physical and chemical processes. A variety of gas-phase air cleaners remove gases using adsorbent media, such as active carbon (AC), to adsorb the pollutants. Other forms of adsorbents are activated aluminium, silica gel, zeolites and organic synthetics (Spry, 2007). Adsorption is a mass transfer process in which gases collide with a solid surface, are attracted to a surface, and remain on the surface. A variety of VOCs can be adsorbed, but the process is typically inefficient for low molecular weight constituents and permanent gases (Daniels, 2007). The adsorption process can be divided into two main groups: physical adsorption (e.g. the adsorption of AC for gas) and chemical adsorption (e.g. activated alumina or AC impregnated with potassium or sodium permanganate, which reacts with formaldehyde and several other compounds) (Fisk, 2007, 2006). Physical adsorption has a very high surface area per unit mass because the adsorbents have extensive microscopic pores, and the process is reversible (i.e. adsorbed VOCs can be released and emitted back into the air). However, the chemical adsorption process is irreversible; consequently, the reacted compound is not subsequently released back into the air.
In general, the adsorption of organic compounds onto carbonaceous adsorbents is primarily controlled by five potential interactions: the hydrophobic effect, - bonds, hydrogen bonds, Van der Waals interactions, and covalent and electrostatic interactions (Wang et al., 2013). Biochar is a kind of carbon material prepared by slow pyrolysis of biomass under an inert atmosphere similar to AC.The production of biochar might result in the release of VOCs, such as methanol, acetic acid, acetone, methyl acetone and acetaldehyde (Tiilikkala et al., 2010). However, biochar has high adsorption efficiency and low cost, so it still has excellent potential for VOC adsorption.
Schieweck (2020) conducted a systematic experimental study dedicated to the removal of museum pollutants. The authors assessed the filtration efficiency of 37 different adsorbent media under both active and passive conditions (with and without forced air exchange). Adsorbents comprised ACs with and without impregnation, including AC cloths, carbon-coated foams, natural and synthetic zeolites, molecular sieves, silica gels, archival cardboard, polymer-impregnated matrixes, and others. The results revealed that the filtration of formaldehyde was challenging for nearly all adsorbents tested. Just 5 out of 37 products exhibited very good (one-pass removal efficiency > 80%) or good performance (removal efficiency > 60%).
Réguer et al. (2011) studied the effect of varying toluene concentrations on the breakthrough from ACs. The breakthrough curves were modelled and experimental made at an inlet concentration 10 times higher than the indoor air level. The results concluded that the henry coefficient (the ratio between the concentration of toluene in ACs at equilibrium and the concentration of toluene in the gas phase at equilibrium) stayed the same at the varying concentration. Weschler et al. (1992) installed panels filled with AC in a test duct with a 0.61 m by 0.30 m cross-section and measured VOC concentrations upstream and downstream of the AC with passive samplers. The air entering the test duct was drawn from indoors; thus, the test duct simulated real deployment. The test duct contained six 2.54-cm-thick carbon-filled panels installed in a zig-zag pattern. The total panel face area was 2.0 m², and the total mass of AC was 20.4 kg (45 lb). The airflow rate through the duct was 0.28 m³/s; thus, the nominal retention time in the carbon bed was 0.18 s, well above the recommended minimum of 0.1 s. The system contained 73 kg of carbon per 1 m³/s of airflow (75 lb per 1000 cfm). The long-term study results of adsorbent performance in a single building demonstrated the initial efficiency and efficiency after 18 months for toluene at 90% and 90%, for p-xylene at 80% and 90%, and for o-xylene at 60% and 70%.
Xiao et al. (2018) developed an in situ thermally regenerated air purifier (TRAP) comprising two chambers and three valves. The switching of the values enabled pollutant adsorption, adsorptive material recycling, and outdoor air intake. Chen et al. (2019) developed a novel flexible adsorption board module with an adjustable surface temperature fabricated with AC, polyimide, and copper foil, as illustrated in Figure 1. Its laminated structure reduced airflow resistance by two orders of magnitude compared with the packed adsorption bed. The built-in copper foil generated Joule heat rapidly and efficiently delivered this heat to the adsorbent, effectively reducing energy consumption. The overall removal efficiency of the fabricated laminated plate was about 30% at the face velocity of 0.8 to 1.2 m/s. The pressure drop was about 5 Pa. Its removal ability can be regenerated in situ in 8 min by increasing the surface temperature to 80°C. The fabricated laminated plate exhibited good durability after 52 cycles of adsorption‐regeneration tests. Chen et al. (2021) proposed a vertical macro-channel modified method to achieve rapid diffusion into the adsorbent during the initial adsorption period. Regular, vertical macro-channels through the adsorption board based on the study by Chen et al. (2019) were fabricated using laser drilling to enhance the mass transfer inside the board. The experimental results demonstrated that, after modification, the penetration times for formaldehyde and xylene extended from 3.8 to 6.2 h and from 62 to 99 h, respectively. The simple macro-channel modification of the adsorption board may be used as an alternative design for adsorption applications in indoor air purification.
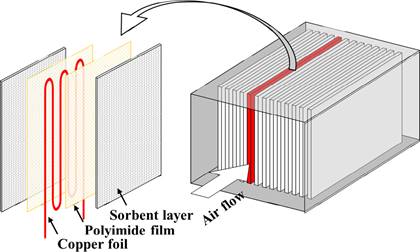
Figure 1. Illustration of the surface temperature adjustable laminated plate structure (left) and the air purifier module comprising multiple parallel plates (right). (Chen et al., 2019)
The study results by Bayer and Hendry (2005) illustrate the difficulties of evaluating adsorbent system performance in uncontrolled field studies. The VOC concentrations can be low, leading to significant measurement errors.
The PCO process is where, upon adsorption of a photon, a semiconductor acts as a catalyst in producing reactive radicals, primarily hydroxyl radicals, which can oxidise organic compounds and mineralise them (Goswami, 2003). Common photocatalysts in PCO are titanium dioxide (TiO2), zinc oxide (ZnO), tungsten trioxide, zinc sulphide, and cadmium sulphide (Gaya and Abdullah, 2008; Tseng et al., 2010). Photocatalytic active coatings made of the semiconductor TiO2 (Mo et al., 2009) have been developed for indoor air application. In its anatase modification, TiO2 has a bandgap of 3.2 eV and can be activated under ultraviolet (UV) light (λ = 387 nm). By light irradiation with a corresponding wavelength, electrons from the valence band are transferred to the conduction band, forming electron-hole pairs (Figure 2). Compared with conventional PCO under UV light (254 or 365 nm), vacuum UV (VUV) light can significantly enhance photocatalytic degradation efficiency. Moreover, by providing a strong oxidation environment and preventing the generation and accumulation of intermediates, VUV light reduces catalyst deactivation (Huang et al., 2017; Shayegan et al., 2017). Despite these benefits, performing PCO with VUV lamps produces O₃ molecules as a by-product. Moreover, O₃ is a powerful oxidising species that can react with VOC pollutants and promote photocatalytic efficiency. However, residual O₃ can damage the environment and human health.
The excited electrons can proceed in a single-electron reduction and, in the presence of O2, form a superoxide radical anion O2 •−. Simultaneously, the electron holes (h+) can react with H2O to yield hydroxyl radicals OH• (single-electron oxidation). These resulting radicals are highly reactive and can degrade a wide range of VOCs and potentially mineralise VOCs into less harmful oxidation products, such as water and carbon dioxide (Héquet, 2018; Mo et al., 2009; Pelaez et al., 2012). The application of TiO2 for photodegradation of organic contaminates has generated significant attention due to its unique characteristics and environmental friendliness (Ji et al., 2017; Tejasvi et al., 2015).
Destaillats et al. (2012) studied the degradation of seven VOCs using a prototype air cleaner provided with flat or pleated PCO filtering media in a 20-m³ stainless-steel chamber at ACH = 1 h−1 under realistic indoor conditions. The media was made of quartz fibres (9 µm in diameter) coated using a sol-gel process with a mixture of 10% to 25% of nanosized TiO2 and 50% to 90% of silicon dioxide and with a BET specific surface area of 120 m² g−1. The authors measured the VOC removal efficiency of PCO air purifiers with airflow from 178 to 878 m³ h−1. The results indicate that the VOC concentration decreased only marginally across the PCO air purifiers at high airflow rates, whereas a decrease in the airflow rate increased the VOC removal efficiency from 5% to 44%. Thus, the PCO cleaning efficiency is not improved when the air recirculation rate is set at higher values.
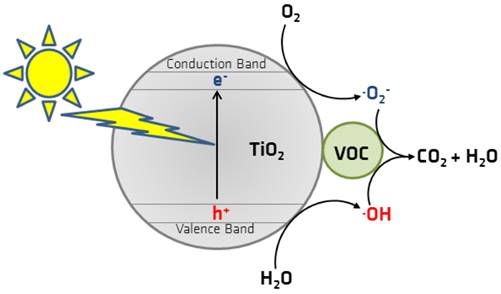
Figure 2. Schematic representation of photocatalytic oxidation of a volatile organic compound (VOC) (Mull et al., 2017).
Zhang and Hsieh (2020) demonstrated a dual-functional polyester fibrous air filter consisting of self-assembled TiO2 nanoparticles and percolated silver nanowires with high air permeability, electrostatic particulate matter removal, and photocatalytic formaldehyde decomposition abilities. With the aid of the decorated photocatalytic TiO2 nanoparticles, the same network can effectively degrade gaseous formaldehyde under UV irradiation.
Air ions are electrically charged molecules or atoms in the atmosphere (Goldstein and Arshavskaya, 1997). An air ion is formed when a gaseous molecule or atom receives sufficiently high energy to eject an electron (Laza, 2000). Negative air ion (NAI) generators gain electrons, whereas positive air ion generators lose electrons. Several types of negative air ion generators are based on corona discharges, thermionic electron emission, photoexcitation, and the Lenard effect for creating NAIs (Lin and Lin, 2017). Among these mechanisms, the corona discharge is an efficient method to generate NAIs. When a high negative voltage is applied to a conductor/electrode and the generated electric field is sufficiently high, corona discharge occurs (Altamimi et al., 2014; Ogar et al., 2017). This type of NAI generator has been commercialised and is the most commonly employed variant. The schematic picture of this technology is presented in Figure 3. Under certain use conditions, ion generator air cleaners can produce levels of O₃ significantly above those thought to be harmful to human health (EPA, 2021).
Wu and Lee (2004) reported that no by-products were generated at a discharge voltage below 16.0 kV. The concentrations of O₃ and nitrogen oxides (NOx) increased with the discharge voltage above 17.0 kV. Therefore, the discharge voltage should be set at 15.0 kV to avoid the generation of O₃ or NOx.
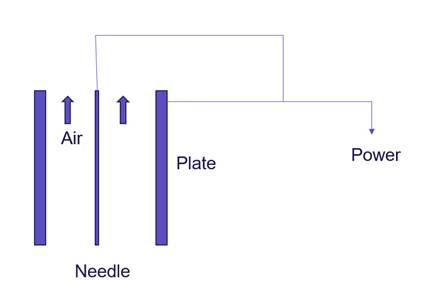
Figure 3. Schematic view of the corona discharge ioniser technology (Rahimi, 2013).
Air ionisation systems have been installed in domestic and office locations to improve indoor air quality. They have also been installed to control volatile compounds and particulates in institutional, commercial and industrial locations.
Daniels (2001) investigated a case study where an air ionisation system was installed in a large engineering centre (Siemens AG, Berlin) with several hundred office workers in a multi-floor facility. Indoor VOCs and O₃ levels were measured continuously in this facility during operational periods with and without air ionisation. The author reported reductions in the levels of 59 specific VOCs representing nine broad classes. For instance, the total VOC (TVOC) level reduced by 50%, and the aromatic substances reduced by 47%. The arithmetic average over one month of operation without air ionisation was 0.7 ppbv, with a maximum of 5.8 ppbv. The arithmetic average over one month of operation with air ionisation was 6.6 ppbv, with a maximum of 14.4 ppbv. The levels in the outside air were not measured directly but were calculated in the range of 10 to 20 ppbv.
Daniels (2001) investigated another case study involving a billing centre near a major international airport (Visa, Zurich) where office workers were subjected to exhaust gases from ground transportation and aeroplane jet engines. Three representative VOCs were quantitated with and without ionisation. The results indicated that the concentration levels of isooctane, benzene and toluene reduced by approximately 35%, 58% and 46%, respectively.
Air ionisation systems have also combined with air filtration to enhance the removal of VOCs and particulates. Tian et al. (2020) proposed and fabricated new electrostatically assisted heterocaking (EAHC) filters using polyurethane (PU) foam with an extremely low pressure drop as base filters and heterogeneously loading high-εr heterocaking (HC) (including manganese dioxide, AC, ZnO, copper oxide, and barium titanate). The schematic of the EAHC air filter module is presented in Figure 4a. Figure 4b presents the schematic of HC fibres in a polarising field compared with standard filter fibres. Some indoor hazardous gases, such as O₃ and formaldehyde, are expected to be removed when the loaded HC fibres are made of an adsorbent or catalyst. The HC filter preparation process is displayed in Figure 4c. The quantitative experiments revealed that the EAHC filter has high single-pass filtration efficiency for airborne PM, O₃, and formaldehyde and has a low pressure drop and low power dissipation. Tian et al. (2021) and Gao et al. (2021) developed new surface coatings on the filtration fibers and dramatically reduced particles and ozone synchronously.
Chen et al. (2020) developed an ioniser-assisted filtration method with an external electrostatic field to efficiently remove gaseous diisobutyl phthalate and dibutyl phthalate. They used low pressure drop PU foam as substrate filters and loaded fine AC powder into PU foam as PU-C foam. The proposed method has developed a new filter based on the existing inexpensive coarse filter, which is easy to implement for the active control of gaseous PAEs.
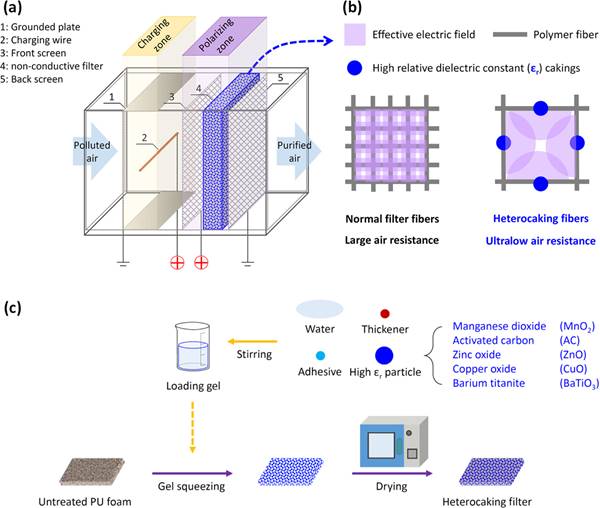
Figure 4. (a) Schematic of the electrostatically assisted heterocaking (EAHC) air filter module, (b) schematic of heterocaking (HC) fibres in a polarising field compared with standard filter fibres, and (c) preparation process of HC filters by a fast and large-scale roll-to-roll gel squeezing method. (Tian et al., 2020).
An O₃ generator is a device that produces O₃ by adding energy to oxygen molecules (O2), which causes the oxygen atoms to separate and temporarily recombine with other oxygen molecules. The process can be accomplished in the following methods: corona discharge and UV radiation. The corona discharge produces O₃ through a method equivalent to lightning, and the UV radiation method is comparable to how the sun’s UV radiation splits O2 molecules to form individual oxygen atoms. Figure 5 illustrates how a corona discharge O₃ generator operates.
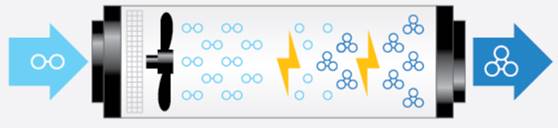
Figure 5. Visualisation of how a corona discharge ozone generator operates (Ozone solutions. 2021). https://ozonesolutions.com/blog/what-is-ozone/
Ozone generators have been used to control indoor air pollution. However, O₃ is associated with adverse health effects, and it is vital to ensure that people and pets are not exposed to high levels of O₃. In addition to the harmful effects of O₃ itself, indoor O₃ can react with building materials, furnishings, and other indoor chemical compounds. Long et al. (2000) studied the indoor chemical reactions involving O₃ and found that the chemical reactions can be a significant source of indoor ultra-fine particles. Shaughnessy et al. (1994) reported that O₃ generators are not effective in removing carbon monoxide. In addition, Esswein and Boeniger (1994) reported that O₃ generators are not effective in removing carbon formaldehyde. Weschler et al. (1992) conducted a laboratory experiment that mixed O₃ with chemicals from new carpets. The authors reported that O₃ reduced many of these chemicals, including those that can produce new carpet odour. However, in the process, the reaction produced a variety of aldehydes, and the total concentration of organic chemicals in the air increased rather than decreased after the introduction of O₃. The reaction rates of O₃ with most VOCs were slow in indoor environments because the characteristic residence times of air and pollutant mixtures in typical indoor settings were too short for the reactions to proceed effectively (Weschler, 2000). Chen et al. (2005) evaluated several air cleaners in the indoor environment and found low VOC removal efficiencies by O₃-based air purifiers and that the indoor O₃ could be at unsafe levels.
Several articles have described air-cleaning plants used by NASA (Wolverton, 1996). Wolverton et al. (1989) found that indoor plants can scrub the air of TVOCs, such as formaldehyde and benzene. Orwell et al. (2004) found that soil microorganisms in potted plants also play a part in cleaning indoor air. In another study, Kim et al. (2010) examined 86 species of houseplants from five general classes for their ability to remove formaldehyde. In their experiments, ferns had the highest formaldehyde-removal efficiency of all the plants tested, especially Osmunda japonica, commonly known as the Japanese royal fern or zenmai. These research studies have positively shown that potted plants could reduce TVOCs from 10% to 90% in 24 h (Llewellyn and Dixon, 2011). In another study, Larsson (2004) examined formaldehyde and TVOCs. The author concluded that the indoor plants reduced formaldehyde by 0.1 to 1.0 mg FAD m−² h−¹ during the daytime, and the reduction for TVOCs was 0.1 to 2 mg TVOC per m−² h−1 during the daytime. The author stated that none of the above-reported effects were considered so critical that they would become an applicable tool in altering indoor air quality.
Middlebrooks (2000) compared pressure drops in three systems with the same mass of granular AC and a 1.0 m/s (200 fpm) face velocity. The reported pressure drops were 38 Pa in the system with 20 to 50 mesh carbon bonded to a pleated nonwoven media, 640 Pa with 20 to 50 mesh carbon in trays, and 75 Pa (0.3-inch H2O) with seven-fold larger 4x8 mesh carbon in trays.
The advantages of PCO are the relatively low pressure drop, ability to treat a wide variety of compounds, and the theoretically long lifecycle of the reactive process. The disadvantages include the electricity used in the lamps and ballasts if fluorescent lamps are used. The power consumed by the largest model is equivalent to 3- to 100-watt standard light bulbs (AiroCide, 2021).
Ozone is produced industrially by bombarding oxygen with UV radiation or passing air through a high-voltage alternate current electrical discharge. Therefore, along with the oxygen requirement, power is also an important consideration. This kind of system typically uses a medium frequency from 800 Hz to 1000 Hz. The O₃ concentrations increase with augmented power. Ozone produced from the air may require up to 15 W/g of O₃, as the power cost of air compression must be included in the cost of O₃ production (Baratharaj, 2013).
Adsorbent-based gas-phase air cleaning is effective for removing a variety of gases, vapours and odours if appropriate types and amounts of adsorbents are used. This removal may impose quite a high pressure drop. The filter replacement interval is of significant importance.
Moreover, PCO has high conversion efficiencies for VOCs at a low pressure drop. However, PCO air cleaners produce O₃ molecules as a by-product. The NAI generator air cleaners can, under certain conditions, produce levels of O₃ and NOx significantly above the levels thought to be harmful to human health.
In addition, available scientific evidence indicates that O₃ is generally ineffective at controlling indoor air pollution at concentrations that do not exceed public health standards. In the process of reacting with chemicals indoors, O₃ can produce other chemicals that can be irritating and corrosive. Many factors affect O₃ concentrations produced by machines that generate O₃, including the amount of O₃ produced by the machines, size of the indoor space, amount of material in the room with which O₃ reacts, outdoor O₃ concentrations and ventilation. These factors make it challenging to control O₃ concentrations.
Altamimi, G., Illias, H. A., Mokhtar, N., Mokhlis, H., and Bakar, A. H. A. Corona discharges under various types of electrodes. In Proceedings of the 2014 IEEE International Conference on Power and Energy (PECon), Kuching Sarawak, Malaysia, 1–3 December 2014; pp. 5–8.
AiroCide. (2021). FAQs. https://kesair.com/faqs/.
ASHRAE Position Document on Filtration and Air Cleaning, ASHRAE, January 29, 2015 Reaffirmed January 13, 2018, https://www.ashrae.org/file%20library/about/position%20documents/filtration-and-aircleaning-pd.pdf.
Baratharaj, V. (2013). How to evaluate and select an ozone generator, OTSIL fact sheets. http://www.otsil.net/articles/HOW-TO-EVALUATE-AND-SELECT-AN-OZONE-GENERATOR.pdf.
Bayer, C. W., and Hendry, R. J. (2005). Field test methods to measure contaminant removal effectiveness of gas phase air filtration equipment – phase 2. ASHRAE Trans. 111(2):p–p.
Chen, C. H., Huang, B. R., Lin, T. S., Chen, I. C., and Hsu, C. L. (2006). A new negative ion generator using ZnO nanowire array. J. Electrochem. Soc. 153G894–G896.
Chen, H., Mo, J., Xiao, R., and Tian, E. (2019). Gaseous formaldehyde removal: A laminated plate fabricated with activated carbon, polyimide, and copper foil with adjustable surface temperature and capable of in situ thermal regeneration. Indoor Air 29, 469–476.
Chen, Q., Liu, F., and Mo, J. (2021). Vertical macro-channel modification of a flexible adsorption board with in-situ thermal regeneration for indoor gas purification to increase effective adsorption capacity. Environ. Res. 192, 110218.
Chen, W., Zhang, J. S., and Zhang, Z. (2005). Performance of air cleaners for removing multiple volatile organic compounds in indoor air. ASHRAE Trans. 111, 1101–1114.
Chen, Z., Tian, E., and Mo, J. (2020). Removal of gaseous DiBP and DnBP by ionizer-assisted filtration with an external electrostatic field. Environ. Poll. 267, 115591.
Destaillats, H., Sleiman, M., Sullivan, D.P., Jacquiod, C., Sablayrolles, J., and Molins, L. (2012). Key parameters influencing the performance of photocatalytic oxidation (PCO) air purification under realistic indoor conditions. Appl. Catal. B Environ. 128, 159–170. https://doi-org.zorac.aub.aau.dk/10.1016/j.apcatb.2012.03.014.
Daniels, S.L. (2001). Applications of Air Ionization for Control of VOCs and PMx. Precision Air, a division of Quality Air of Midland, Inc. https://www.plasma-air.com/resources/397.
Daniels, S.L. (2007). On the qualities of the air as affected by radiant energies (photocatalytic ionization processes for remediation of indoor environments). J. Environ. Eng. Sci. 6, 329–342.
Esswein, E. J., and Boeniger, M. F. (1994). Effects of an ozone-generating air-purifying device on reducing concentrations of formaldehyde in air. Appl. Occup. Environ. Hygiene 9(2), 139 –146.
Fisk, W. J. (2007). Can sorbent-based gas phase air cleaning for VOCs substitute for ventilation in commercial buildings, in Proceedings of IAQ 2007, Baltimore, Maryland. https://escholarship.org/content/qt4ww2d5wv/qt4ww2d5wv.pdf.
Fisk, W. J. (2006). Sorbent-based gas phase air cleaning for VOCs in commercial buildings. U.S. Department of Energy Office of Scientific and Technical Information. doi:10.2172/898957. https://www.osti.gov/servlets/purl/898957-jFF50W/.
Goswami, D. Y. (2003). Decontamination of ventilation systems using photocatalytic air cleaning technology. Trans. ASME. J. Solar Energy Eng. 125, 359–365.
Gao, Y., Tian, E., Mo, J. (2021). Electrically responsive coarse filters endowed by high-dielectric-constant surface coatings toward efficient removal of ultrafine particles and ozone. ACS ES&T Engg. https://doi.org/10.1021/acsestengg.1c00186.
Gaya, U. I., and Abdullah, A. H. (2008). Heterogeneous photocatalytic degradation of organic contaminants over titanium dioxide: a review of fundamentals, progress and problems. J. Photochem. Photobiol. C 9 1, 1–12.
Goldstein, N., and Arshavskaya, T. V. (1997). Is atmospheric super oxide Vitally necessary? Accelerated death of animals in a quasi-neutral electric atmosphere. Z. Naturforsch C. 52, 396–404. doi: 10.1515/znc-1997-5-619.
Ji, J. et al. (2017). Mesoporous TiO2 under VUV irradiation: enhanced photocatalytic oxidation for VOCs degradation at room temperature. Chem. Eng. J. (Supplement C) 327, 490–499.
Huang, H., Huang, H., Liu, G., Zhan, Y., Xu, Y., Lu, H., et al. (2017). Photocatalytic oxidation of gaseous benzene under VUV irradiation over TiO2/Zeolites catalysts, Catal. Today (Part 3) 281, 649–655.
Héquet, V., Raillard, C., Debono, O., Thévenet, F., Locoge, N., and Le Coq, L. (2018). Photocatalytic oxidation of VOCs at ppb level using a closed-loop reactor: The mixture effect. Appl. Catal. B: Environ. 226, 473–486.
Kim, K. J., Jeong, M., Lee, D. W., Song, J. S., Kim, H. D., Yoo, et al. (2010). Variation in formaldehyde removal efficiency among indoor plant species. Hortscience 45(10), 1489–1495.
Larsson, T. (2004). Influence of some indoor plants on the indoor air quality. [PhD thesis]. [Gothenburg, Sweden] Department of Building Technology, Chalmers University of Technology.
Laza, V. (2000). The environment and gaseous ions. Cent. Eur. J. Occup. Environ. Med. 6, 3–10.
Lin, H. F., and Lin, J. M. (2017). Generation and determination of negative air ions. J. Anal. Test. 1, 6.
Mull, B., Möhlmann, L., and Wilke, O. (2017), Photocatalytic degradation of toluene, butyl acetate and limonene under UV and visible light with titanium dioxide-graphene oxide as photocatalyst. Environments 4(1), 9. doi:10.3390/environments4010009.
Long, M., Suh, H., and Koutrakis, P. (2000). Characterization of indoor particle sources using continuous mass and size monitors. J. Air Waste Manag. Assoc. 50, 1236–1250.
Llewellyn, D., and Dixon, M. (2011). “Can Plants Really Improve Indoor Air Quality? Reference Module in Life Sciences,” in Comprehensive Biotechnology, 2nd ed., vol. 4; (Amsterdam, The Netherlands: Elsevier), 331–337.
Li Puma, G., Salvadó-Estivill, I., Obee, T. N., and Hay, S. O. (2009). Kinetics rate model of the photocatalytic oxidation of trichloroethylene in air over TiO2 thin films. Sep. Purif. Technol. 67, 226–232.
Middlebrooks, M. C. (2000). Benefits of non-woven structure on adsorption performance of novel filter medium. Proceedings of Techtextil North American Symposium, Atlanta, GA.
Mo, J., Zhang, Y., Xu, Q., Lamson, J. J., and Zhao, R. (2009). Photocatalytic purification of volatile organic compounds in indoor air: A literature review. Atmos. Environ. 43, 2229–2246.
Ogar, V. N., Bendor, S. A., and James, A. E. (2017). Analysis of corona effect on transmission line. Am. J. Eng. Res. 6, 75–87.
Orwell, R. L., Wood, R. L., Tarran, J., Torpy, F., and Burchett, M.D. (2004). Removal of benzene by the indoor plant/substrate microcosm and implications for air quality. Water, Air, Soil Poll. 157, 193–207.
Ozone Solutions. (2021). What is Ozone? https://ozonesolutions.com/blog/what-is-ozone/.
Peterson, M. S., Zhang, W., Fisher, T. S., and Garimella, S. V. (2005). Low-voltage ionization of air with carbon-based materials. Plasma Sources Sci. Technol. 14, 654–660.
Pelaez, M., Nolan, N. T., Pillai, S. C., Seery, M. K., Falaras, P., Kontos, et al. (2012). A review on the visible light active titanium dioxide photocatalysts for environmental applications. Appl. Catal. B Environ. 125, 331–349.
Réguer, A., Sochard, S., Hort, C., and Platel, V., (2011). Measurement and modelling of adsorption equilibrium, adsorption kinetics and breakthrough curve of toluene at very low concentrations on to activated carbon. Environ. Technol. 32, 757-766.
Richardson, G., Eick, S. A., Harwood, D. J., Rosen, K. G., and Dobbs, F. (2003). Negative air ionisation and the production of hydrogen peroxide. Atmos. Environ. 37, 3701–3706.
Shayegan, Z., Chang-Seo Lee, C. S., and Haghighat, F. (2018). TiO₂ photocatalyst for removal of volatile organic compounds in gas phase–A review. Chem. Eng. J. 334, 2408–2439.
Shaughnessy, R. J., Levetin, E., Blocker, J., and Sublette, K. L. (1994). Effectiveness of portable indoor air cleaners: sensory testing results. Indoor Air. J. Int. Soc. Indoor Air Quality Clim. 4, 179–188.
Spry, P. (2007). “The science of gas phase air cleaning,” In Proceedings of Clim. 2007 WellBeing Indoors, vol. A03. https://www.researchgate.net/publication/229015153_The_Science_of_Gas_Phase_Air_Cleaning.
Schieweck, A. (2020). Adsorbent media for the sustainable removal of organic air pollutants from museum display cases. Herit. Sci. 8, 12. https://doi.org/10.1186/s40494-020-0357-8.
Tian, E., Xia, F., Wu, J., Zhang, Y. Li, J., Wang, H., and Mo, J. (2020). Electrostatic air filtration by multifunctional dielectric heterocaking filters with ultralow pressure drop. ACS Appl. Mat. Interfaces 12(26), 29383–29392.
Tian, E., Yu, Q., Gao, Y., Wang, H., Wang, C., Zhang, Y., Li, B., Zhu, M., Mo, J., Xu, G., and Li, J. (2021). Ultralow resistance two-stage electrostatically assisted air filtration by polydopamine coated pet coarse filter. Small, 2102051.
Tiilikkala, K., Fagernäs, L., and Tiilikkala, J. (2010). History and use of wood pyrolysis liquids as biocide and plant protection product. The Open Agricult. J. 4, 111–118.
Tseng, T. K., et al. (2010). A review of photocatalysts prepared by sol-gel method for VOCs removal. Int. J. Mol. Sci. 11(6), 2336.
Tejasvi, R., Sharma, M., and Upadhyay, K. (2015). Passive photo-catalytic destruction of airborne VOCs in high traffic areas using TiO2-coated flexible PVC sheet. Chem. Eng. J. (Supplement C) 262, 875–881.
United States Environmental Protection Agency (EPA). (2021). Ozone generators that are sold as air cleaners. www.epa.gov/indoor-air-quality-iaq/ozone-generators-are-sold-air-cleaners.
Wang, S., Sun, H., Ang, H. M., and Tadé, M. O. (2013). Adsorptive remediation of environmental pollutants using novel graphene-based nanomaterials. Chem. Eng. J. 226, 336–347.
Weschler, C. J. (2000). Ozone in indoor environments: concentration and chemistry. Indoor Air 10, 269–288.
Weschler, C. J., Hodgson, A. T., and Wooley, J. D. (1992). Indoor chemistry: Ozone, volatile organic compounds and carpets. Environ. Sci. Technol. 26(12), 2371–2377.
Wolverton, B. C. (1996). Eco-friendly Houseplants, 50 Indoor Plants that Purify the Air in Homes and Offices, London: Weidenfeld & Nicolson, 0-297-83484-3.
Wolverton, B. C., Douglas, W. L., and Bounds, K. (1989). A study of interior landscape plants for indoor air pollution abatement. Technical Memorandum (TM). Document ID: 19930072988. https://ntrs.nasa.gov/api/citations/19930072988/downloads/19930072988.pdf
Zhang, R.-Y., and Hsieh, G.-W. (2020). Electrostatic polyester air filter composed of conductive nanowires and photocatalytic nanoparticles for particulate matter removal and formaldehyde decomposition. Environ. Sci.: Nano 7(12), 3746–3758.
Follow us on social media accounts to stay up to date with REHVA actualities
0